Olympus Announces New Endobronchial Ultrasound (EBUS) Bronchoscope
The BF-UC190F Aids Physicians in Diagnosing and Staging Lung Cancer without Surgery

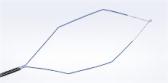
The BF-UC190F EBUS bronchoscope is the newest addition to the robust Olympus EBUS portfolio of devices for the minimally invasive diagnosis and staging of lung cancer.
CENTER VALLEY, Pa., (May 12, 2021) – Olympus announced today the market launch of the FDA 510(k)-cleared BF-UC190F endobronchial ultrasound (EBUS) bronchoscope, the newest addition to its robust EBUS portfolio of devices for minimally invasive lung cancer diagnosis and staging via needle biopsy. Today, more than 1,600 clinical cases using the Olympus EBUS solution prove that EBUS-TBNA (transbronchial needle aspiration) is an effective biopsy solution, providing higher diagnostic yield with greater sensitivity and specificity in lymph nodes ≥5 mm. According to the American College of Chest Physicians (ACCP), correctly staging lung cancer is critical, because the treatment options and prognosis differ significantly by stage, and the ACCP recommends EBUS-TBNA as the optimal first test rather than surgery for mediastinal staging.i
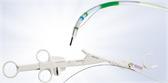
In conjunction with the versatile Olympus ViziShot EBUS-TBNA needle portfolio, the BF-UC190F allows physicians to access and sample difficult-to-reach targets such as lymph nodes in the mediastinum (4L) and hilum (10R). Key design features of the BF-UC190F that enable improved access and sampling include:
- Powerful Scope Angulation: With an increase in angulation from 120 degrees to 160 degrees, this new EBUS-TBNA bronchoscope has the flexibility to ensure the correct positioning of the scope tip relative to the target.
- Compact Distal Tip: With a distal diameter of 6.6mm, the BF-UC190F is smoother to insert and easier to maneuver within the lung.
- Steeper Puncture Angle: With a more perpendicular needle orientation, this allows for smoother penetration between cartilage rings and other critical anatomy.
- Enhanced Endoscopic View: With a decreased forward oblique angle of 20 degrees and higher resolution images, the BF-UC190F increases the field of view and improves visualization of the anatomy for more precise airway navigation and abnormality detection.
“When reaching a lymph node to obtain a tissue sample, it is critical to be able to achieve the correct positioning within the lung. In my study of this new EBUS-TBNA bronchoscope, I found that the sharper puncture angle greatly improves the ability of physicians to puncture the lymph node precisely without needing to use more force or to alter technique,” said Kazuhiro Yasufuku, MD, PhD, FRCSC, Professor and Chair of Thoracic Surgery at the University of Toronto and the William Coco Chair in Surgical Innovation for Lung Cancer at the University Health Network. “The BF-UC190F bronchoscope is a valuable advancement in the use of EBUS-TBNA for the diagnosis and staging of lung cancer.”
“We are very excited to launch this next-generation EBUS bronchoscope, which will further the ability of physicians to biopsy mediastinal and hilar lymph nodes through a minimally invasive procedure,” said Lynn Ray, Vice President and General Manager of the Global Respiratory Business Unit for Olympus Corporation. “Our goal as a company is to develop leading-edge technology that helps physicians diagnose disease earlier and more accurately, which can save lives.”
According to the American Cancer Society, lung cancer is the second most common cancer and the leading cause of cancer death in the US — in 2021, an estimated 235,760 people were diagnosed with lung cancer, and 131,880 people died of the disease.ii While lung cancer has an overall five-year survival rate of only 20 percent, lung cancer detected and treated at an early stage increases the chance of survival beyond five years.iii In March 2021, the U.S. Preventive Services Task Force (USPSTF) announced new recommendations for annual lung cancer screening using low-dose computed tomography (LDCT) for people at high risk for lung cancer, lowering the screening age to 50 and lowering the smoking threshold to 20 pack years, or those who currently smoke or have quit within the past 15 years.iv
For more information about the BF-UC190F, please visit https://medical.olympusamerica.com/products/bf-uc190f
# # #
About Olympus
As leading medical technology company, Olympus uses innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures to improve clinical outcomes, reduce overall costs, and enhance the quality of life for patients. Olympus’ Medical portfolio includes endoscopes, laparoscopes, and video imaging systems, as well as surgical energy devices, system integration solutions, medical services, and a wide range of endotherapy instruments. For more information, visit https://medical.olympusamerica.com.
- Frank C. Detterbeck FC, Postmus, PE, Tanoue, LT. Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. CHEST. 2013; 143(5).
- Key Statistics for Lung Cancer. Cancer.org. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html Last revised January 12, 2021. Accessed May 7, 2021.
- Non-Small Cell Lung Cancer Treatment (PDQ®)–Health Professional Version. National Cancer Institute. Updated November 18, 2020. Accessed January 15, 2021. https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq
- Lung Cancer: Screening. USpreventiveservicestaskforce.org. Published March 9,2021. Accessed April 21, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening.