Olympus Announces U.S. Launch of the SecureFlex™ Single-Use Fine Needle Biopsy Device
Olympus’ Newest Needle for Endoscopic Ultrasound-Guided Biopsy is Designed to Offer Precision Sampling When Access Matters Most
CENTER VALLEY, Pa. (Jan. 28, 2026) – Olympus Corp. of the Americas, a global MedTech company committed to making people’s lives healthier, safer and more fulfilling, announced today the U.S. launch of its most advanced single-use fine needle biopsy device used in ultrasound-guided tissue sampling for the diagnosis of diseases such as pancreatic cancer.
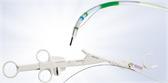
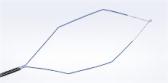
The SecureFlex™ FNB needle is designed to enable the collection of large, intact tissue samples and provide easy access to difficult-to-reach lesions in areas such as the pancreatic head and the uncinate process. Olympus offers the SecureFlex needle in 19G, 22G and 25G sizes.
Olympus will highlight the SecureFlex FNB needle during the Cedars-Sinai Endoscopy Symposium Jan. 28-31 in Los Angeles and Orlando Live Endoscopy 2026 Feb. 4-6 in Orlando.
EUS-FNB: Endoscopic Ultrasound-guided Fine Needle Biopsy
EUS-FNB is a technique that combines ultrasound and endoscopy technologies, enabling physicians to obtain tissue or cell biopsies of lesions that cannot be accessed directly by the endoscope. A needle biopsy, for example, may be performed through the wall of the gastrointestinal tract with the ultrasound scope being inserted orally and ultrasound imaging used for sub-mucosal visualization.
“Gastroenterologists require precision and reliability when performing a biopsy for diagnostic accuracy. Olympus is excited to offer U.S. customers a dependable biopsy solution designed for precision sampling,” said Christian Hagie, Vice President, GI EndoTherapy Business Unit Leader, Olympus Corporation of the Americas. “The SecureFlex EUS-FNB needle offers an advanced design to help physicians perform efficient procedures by facilitating access to complex anatomical structures and obtain adequate samples for diagnostic testing, even in challenging cases.”1
Precision Sampling
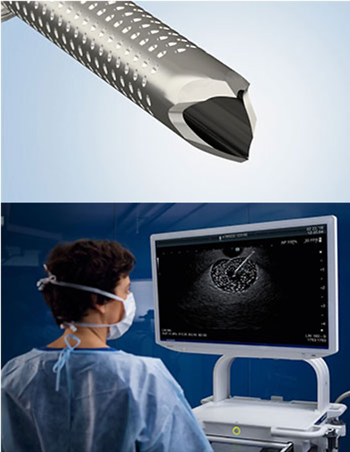
The SecureFlex’s Dual-Beveled Raptor™ Tip is designed with an outer distal cutting surface that forms a fine edge to support smooth tissue puncture. The inner proximal cutting surface then guides more tissue into the needle. Compared to its predecessor, the tip of the SecureFlex needle helps preserve cellular architecture through precise tissue execution.1
Other features of the SecureFlex EUS-FNB needle include:
- Nitinol construction* which can prevent needle deformation in tortuous anatomy and maintains straightness even after multiple passes1
- Multi-layer sheath design for effortless handling to allow for smooth operation2
- Houndstooth-pattered dimpling for enhanced ultrasound visibility.
In recent years, advances in the treatment of pancreatic cancer — including molecularly targeted therapies and immunotherapies — have increased the need for high-quality tissue sampling. For these therapies, it is often critical to obtain larger and higher-quality samples for accurate treatment decisions.
The SecureFlex EUS-FNB needle is part of a larger GI portfolio that includes the EU-ME3 Ultrasound Processor, which provides image quality and functionality that is comparable to a high-end ultrasound center but in a compact body.3 The EU-ME3 supports diagnostic imaging and ultrasound-guided intervention for hepatobiliary-pancreatic procedures and expands the boundaries of endosonography with advanced functions and high-resolution imaging.
EUS Needle Risks: Complications from extra-luminal EUS guided FNB may include, but are not limited to infection, bleeding, perforation, and/or tumor seeding. Extra-luminal fine needle aspiration of cystic lesions has a higher risk of complication from infection and hemorrhage.
For more information about the entire Olympus GI portfolio, visit the gastroenterology product page.
# # #
About Olympus
At Olympus, we are committed to Our Purpose of making people’s lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states.
Olympus Corporation of the Americas, a wholly owned subsidiary of Olympus Corporation, is headquartered in Center Valley, Pennsylvania, USA, and employs more than 4,500 employees throughout locations in North and South America. For more information, visit olympusamerica.com.
* Nitinol construction refers to 19G and 22G sizes
1 Data on file with Olympus (DC01134422)
2 Data on file with Olympus (DC01088737)
3 Data on file with Olympus (DC00719472)