Olympus EU-ME3 Ultrasound Processor for GI and Pulmonary Disease Detection and Diagnosis Launches in U.S. Market
CENTER VALLEY, Pa., (July 16, 2025) – Olympus Corp., a global medical technology company committed to making people's lives healthier, safer, and more fulfilling, announced today the U.S. launch of the next-generation EU-ME3 Ultrasound Processor, which can integrate endoscopic and endobronchial ultrasound (EUS/EBUS) on a single workstation. The EU-ME3 provides image quality and functionality that is comparable to a high-end ultrasound center but in a compact body.i
The versatile EU-ME3 supports diagnostic imaging and ultrasound-guided intervention for hepatobiliary-pancreatic and pulmonary procedures and expands the boundaries of endosonography with advanced functions, high-resolution imaging and user-friendly operation.
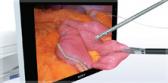
Supporting the detection and characterization of lesions, the EU-ME3 delivers high image quality for clear visualization in EUS and EBUS procedures. Imaging technologies, such as s-FOCUS and Tissue Harmonic Echo, allow for focusing throughout the depth of field, improved resolution and signal-to-noise ratio, and fewer artifacts.i
Smart user settings let health care providers customize configurations to suit physician needs and procedural requirements.
Backward and forward universal compatibility with Olympus® EUS and EBUS endoscopes and miniature probes makes this ultrasound processor adaptable and can help facilities maximize cost efficiencies across specialties.i
“As healthcare facilities look for ways to improve patient outcomes and save costs, we are pleased to offer our customers a versatile, state-of-the-art ultrasound platform for GI and pulmonary applications,” said Richard Reynolds, President of the Medical Systems Group at Olympus Corporation of the Americas. “The EU-ME3 allows physicians to realize clinical efficacy through premier imaging that supports early detection and diagnosis for patients.”
Gastrointestinal EUS
Endoscopic ultrasound (EUS) combines ultrasound technology with endoscopy to visualize the tissues of the upper digestive tract and adjacent anatomical structures inside the human body. With EUS, the transducer is endoscopically inserted into the body via the mouth, positioning it close to the area of interest to obtain high resolution images.
The EU-ME3 Ultrasound Processor provides clear visualization and assists in diagnostic, therapeutic and interventional procedures for cancers and diseases of the upper GI tract and surrounding organs, including the pancreas, bile duct, liver, spleen and gallbladder.
According to the American Cancer Society, the estimated number of newly diagnosed cases of upper GI cancers will be more than 197,000 in the U.S. for 2025, accounting for an estimated 119,590 deaths.ii Visualization tools like EUS support physicians in detecting and diagnosing GI cancers at their earliest stages when patients have the greatest chance of survival.
Pulmonary EBUS
The system also offers support for endobronchial ultrasound (EBUS) procedures. EBUS is a minimally invasive medical procedure that allows doctors to look at the lymph nodes adjacent to the airways. Olympus’ EU-ME3 offers both linear and radial EBUS capabilities in one ultrasound processor for enhanced procedural and cost efficiencies.
American College of Chest Physicians (CHEST) Lung Cancer Guidelines recommend EBUS-TBNA (transbronchial needle aspiration) over surgical staging for mediastinal staging of lung cancer as a best first test.iii EBUS-TBNA offers a nonsurgical solution using real-time ultrasound-guided tissue sampling. In addition, radial EBUS is recommended as an adjunct imaging modality for patients who have a peripheral lung nodule and for whom a tissue diagnosis is required due to uncertainty of diagnosis or poor surgical candidacy.iii EBUS-TBNA and radial EBUS support procedures for early, minimally invasive diagnosis and lung cancer staging.
According to the National Cancer Institute, lung cancer is the leading cause of all cancer-related deaths in the United States and is estimated to claim almost 125,000 lives in 2025.iv Despite there being recommended screening guidelines, only 16% of those identified as being at high risk are screened each year. The survival rate for lung cancer diagnosed at its earliest and most curable stage is 67% as compared to 12% for lung cancer caught at a later stage after it has spread to other regions of the body.v
Advancing Endosonography
This next-generation processor also offers these advanced endosonographic features, which can be added to the system according to user needs and budget, at any time.
- Shear Wave Quantification (SWQ): Provides an absolute value of tissue stiffness within a region of interest. It performs this quantitative tissue assessment by calculating the propagation velocity of shear waves generated from a push-pulse.i
- Elastography (ELST): Visualizes the amount of strain in the tissue (tissue stiffness) during compression and retraction, making it possible to obtain more information about tissue properties.i
- Contrast Harmonic Echo (CHE): Images harmonic components from ultrasound contrast agents. The newly added C-THE mode derives images from signals produced by biological tissue and the contrast agent, simultaneously.i
For more information or to evaluate the EU-ME3, please visit the EU-ME3 Ultrasound Processor product page.
# # #
About Olympus
At Olympus, we are committed to Our Purpose of making people’s lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states.
Olympus Corporation of the Americas, a wholly owned subsidiary of Olympus Corporation, is headquartered in Center Valley, Pennsylvania, USA, and employs more than 4,500 employees throughout locations in North and South America. For more information, visit olympusamerica.com.
i Data on file with Olympus as of January 2025
ii Cancer Facts & Figures, 2025. Cancer.org. Published June 2025. Accessed July 7, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2025/2025-cancer-facts-and-figures-acs.pdf
iii Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC: “Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines” CHEST 2013; 143(5)(Suppl):e211S–e250S.
iv Cancer State Facts: Lung and Bronchus Cancer. Seer.cancer.gov. Accessed July 3, 2025. https://seer.cancer.gov/statfacts/html/lungb.html
v Lung Cancer Survival Rates. Cancer.org. Last revised: June 27, 2025. Accessed July 3, 2025. https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging/survival-rates.html