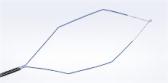
Olympus Receives 510(k) Clearance for TJF-Q180V Duodenoscope
CENTER VALLEY, Pa., (January 15, 2016) – Olympus announced today that FDA has cleared the 510(k) for the TJF-Q180V duodenoscope with modifications to the device design and labeling. The duodenoscope is a flexible gastrointestinal endoscope used in procedures such as endoscopic retrograde cholangiopancreatography (ERCP), which is most commonly performed to diagnose and treat conditions in the pancreas, bile duct and gallbladder.
Beginning in early February, Olympus will implement a corrective action in the U.S. for current TJF-Q180V duodenoscopes. These changes involve new parameters that were reviewed by FDA prior to 510(k) clearance. The corrective action includes replacing the forceps elevator mechanism with a new forceps elevator design.
Detailed instructions about how to complete this process will be sent to customers via Federal Express on January 15, 2016. In the meantime, more information can be found here. Healthcare facilities should continue to use the TJF-Q180V duodenoscope until the forceps elevator mechanism is replaced.
Additionally, the corrective action includes an updated Operation Manual and Reprocessing Manual for the TJF-Q180V. The Operation Manual contains new information on the required inspection of the device before and after patient procedures that must be performed by the healthcare facility. The Reprocessing Manual includes updated reprocessing instructions, including information on the use of Acecide-C High-Level Disinfectant when disinfecting the TJF-Q180V in the OER-Pro and a new chapter (Chapter 6) regarding annual inspections by Olympus service personnel.
The new reprocessing measures should be implemented as soon as possible. Healthcare facilities can download the new manuals from the OlympusConnect website. New printed manuals will be available for distribution in February.
Olympus intends to implement the replacement of the forceps elevator mechanism and provide updated manuals in all other markets with this device in the coming months.
# # #
Distributed by Olympus Corporation of the Americas
About Olympus
Olympus is a precision technology leader, designing and delivering innovative solutions in its core business areas of Medical and Surgical Products, Scientific Solutions, and Cameras and Audio Recorders. Through this technology, Olympus focuses on enhancing people’s lives every day and helping people enjoy the continuum of life. For more information, visit www.olympusamerica.com.