Spiration® Valve System for Emphysema
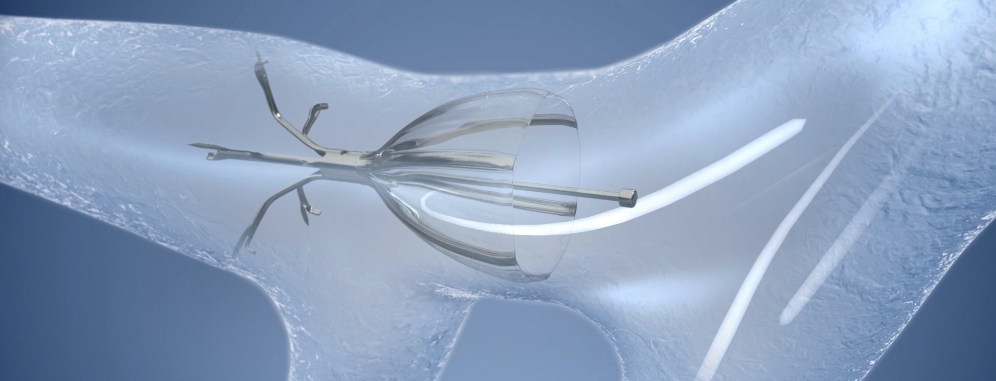
The Spiration Valve System is an innovative endobronchial therapy that offers patients with severe emphysema a customized, minimally invasive treatment option for lung volume reduction with a favorable risk-benefit profile. Patients treated with the Spiration Valve System in clinical trials experienced improvements in breathlessness, lung function, and quality of life.1
The Right Patient
SeleCT® | The Key to Successful Patient Outcomes
Proper patient selection is crucial for the success of endobronchial valve treatment to achieve a good response to therapy. SeleCT is an artificial intelligence (AI) based patient screening platform using a quantitative CT analysis service that is completely non-invasive and offers not only rapid results but also includes a qualified overread by a trained medical professional. This solution makes additional, more invasive assessment tests unnecessary.
Key quantitative measures to identify responders for the Spiration Valve System:
Emphysema Severity
Allow physicians to quickly identify the most diseased lobe.Heterogeneity
Differentiates target and ipsilateral lobe emphysema to facilitate redirection of ventilation to healthier tissue.Fissure Integrity
EMPROVE trial results confirmed radiographic assessment of fissure completeness to be a reliable surrogate for collateral ventilation.
The Right Valve
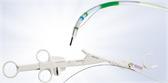
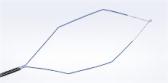
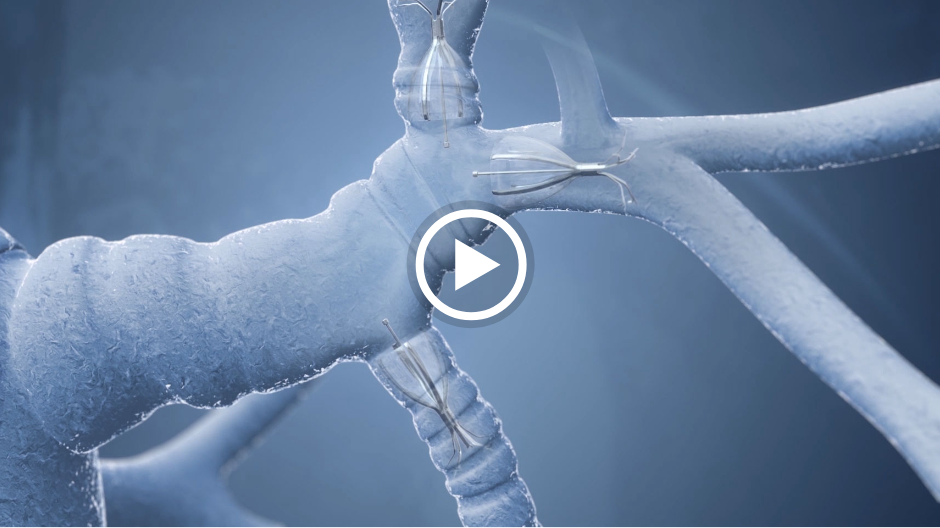
How the Spiration Valve Works
The unique umbrella-shaped design of the Spiration Valve redirects air from diseased parts of the lung to healthier parts, all while allowing trapped air and secretions to escape naturally along the airway wall, so that patients may breathe easier.
Why Choose the Spiration Valve System?
A Decade of Clinical Experience
The Spiration Valve System has been available in the US Market, since 2008, under a Humanitarian Device Exemption (HDE) FDA approval, for the management of post-surgical prolonged air leaks. Physicians continue to choose the Spiration Valve System for emphysema treatment because of their trust and familiarity of the product after years of clinical practice with air leaks.

I choose the Spiration Valve System because I have experience with it. I know how it works, how to deploy it, how it sits, and how to take it out, if necessary.”
Confidence in Valve Placement
The Spiration Valve System anchor design allows for more flexibility as to where to deploy the valves, independent of airway depth or access to a carina. This unique valve minimizes contact with the bronchial wall, maintains position to redirect air even in complex airways and facilitates removal when needed.
Potential complications which may be associated with bronchoscopy and/or the Spiration Valve System may include, but are not limited to, pneumothorax, worsening of COPD symptoms, pneumonia, dyspnea and in rare cases, death. Prior to using the Spiration Valve System, please review the full list of prescriptive information at https://svs.olympusamerica.com/prescriptive-information for additional information on indications, contraindications, warnings, precautions and potential complications.
The Right Outcomes
Benefits That Make an Impact on Patient Lives
The EMPROVE Clinical Trial demonstrated the Spiration Valve System is a safe and effective treatment option for patients with severe emphysema and little-to-no collateral ventilation.1

Low 14.2% rate of serious pneumothorax
Short 24 min. procedure time which may reduce the risk of serious adverse events1,5
52.8% reduction of the target lobe volume


6.3% reduction in hyperinflation
As measured by RV/TLC ratio
12.1% significant increase in FEV1


29.6% decrease in dyspnea
as determined by mMRC score
9.5 point reduction in SGRQ* score
Demonstrating a substantial and clinically-meaningful improvement

Potential complications which may be associated with bronchoscopy and/or the Spiration Valve System may include, but are not limited to, pneumothorax, worsening of COPD symptoms, pneumonia, dyspnea and in rare cases, death. Prior to using the Spiration Valve System, please review the full list of prescriptive information at https://svs.olympusamerica.com/prescriptive-information for additional information on indications, contraindications, warnings, precautions and potential complications.

Recommended by GOLD
The Spiration Valve System received an Evidence A Rating from the Global Initiative for Chronic Obstructive Lung Disease (GOLD)2, affirming that endobronchial valves are a viable minimally invasive treatment option for severe emphysema.
Alpha-1 Antitrypsin Deficiency
The Only Valve Indicated for this Genetic Condition
Alpha-1 antitrypsin deficiency is a genetic condition and can often result in Chronic Obstructive Pulmonary Disease (COPD) and severe emphysema symptoms in patients of any age. The Spiration Valve has been studied and shown to benefit these patients. Spiration Valve placement resulted in improvements in lung function, quality of life, and shortness of breath.1
- Improvement in
Lung Function - Target Lobe
Volume Reduction - Improvements in Health Status, Dyspnea
and COPD Assessment
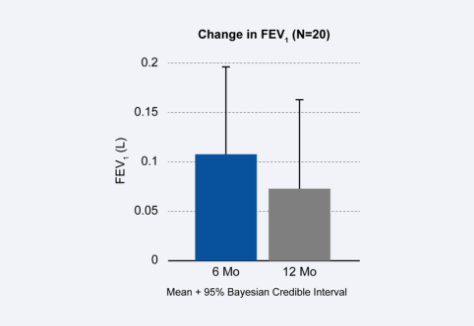
Potential complications which may be associated with bronchoscopy and/or the Spiration Valve System may include, but are not limited to, pneumothorax, worsening of COPD symptoms, pneumonia, dyspnea and in rare cases, death. Prior to using the Spiration Valve System, please review the full list of prescriptive information at https://svs.olympusamerica.com/prescriptive-information for additional information on indications, contraindications, warnings, precautions and potential complications.
Patient Stories
Real Results from Real Patients

“[This procedure] is marvelous. It changes your life…it is a second chance at life.”
Watch Lucie’s Story
“A few days afterwards, I started feeling normal again, like a mother, a grandmother, and just getting back to doing things that I love to do.”
Watch Marion’s Story
“I can walk up the stairs, and I’m not out of breath so frequently. I had a major improvement on my life.”
Watch Ronny’s Story
“The term ‘life-changing’ comes to mind. At least for me, I’ve gotten a lot back that I had lost.”
Watch Chris and Jodi’s StoryPotential complications which may be associated with bronchoscopy and/or the Spiration Valve System may include, but are not limited to, pneumothorax, worsening of COPD symptoms, pneumonia, dyspnea and in rare cases, death. Prior to using the Spiration Valve System, please review the full list of prescriptive information at https://svs.olympusamerica.com/prescriptive-information for additional information on indications, contraindications, warnings, precautions and potential complications.
Frequently Asked Questions
- Non-serious (Moderate) PTX led to Respiratory failure
- Serious PTX. Pneumonia occurred 7 days later
- Non-serious (Severe) PTX associated with pneumonia
- Non-serious (Moderate) associated with respiratory failure
- Serious PTX associated with respiratory failure
- Non-serious (Moderate) led to pneumonia 7 days later
- Serious PTX associated with pneumonia
| Delta Baseline – 3 Months | FEV1 (ml) | mMRC (points) | CAT (points) | SGRQ (points) |
|---|---|---|---|---|
| Serious PTX (N=14) | 129 ±222 | -0.62 ±1.26 | -4.3 ±7.5 | -10.3 ±15.2 |
| No PTX (N=99) | 124 ±164 | -0.49 ±0.96 | -3.0 ±7.0 | -8.2 ±17.8 |
| P- value between groups | 0.643 | 0.921 | 0.893 | 0.802 |
| UPPER LOBES | 9mm valves only | 9 + mix of valves | No 9mm valves |
|---|---|---|---|
| Serious PTX | 4/22 | 9/35 | 6/22 |
| Non-serious PTX | 3/22 | 2/35 | 1/22 |
| LOWER LOBES | 9mm valves only | 9 + mix of valves | No 9mm valve |
| Serious PTX | 1/10 | 0/20 | 0/4 |
| Non-serious PTX | 1/10 | 6/20 | 0/4 |
| ALL LOBES | 9mm valves only | 9 + mix of valves | No 9mm valves |
| Serious PTX | 5/32 | 9/55 | 6/26 |
| Non-serious PTX | 4/32 | 8/55 | 1/26 |
| Measure | MCID | EMPROVE | LIBERATE |
|---|---|---|---|
| SGRQ | 4.0 points | 50.5% | 56.2% |
| mMRC | 1.0 point | 48.9% | 47.8% |
| Delta Baseline – 6 Months | FEV1 (ml) | TLV (L) | RV (L) | mMRC (points) | SGRQ (points) |
|---|---|---|---|---|---|
| Fissure Integrity ≥95% (N=87) | 112 ±159 | -1.02 ±0.75 | -0.49 ±0.85 | -0.66 ±1.04 | -9.5 ±16.7 |
| Fissure Integrity 90-94% (N=19) | 38 ±115 | -0.74 ±0.60 | -0.002 ±0.75 | -0.58 ±1.02 | -1.9 ±18.0 |
| P- value between groups | 0.0576 | 0.1607 | 0.0224 | 0.7601 | 0.0853 |
PulmonX makes this claim based on a retrospective analysis of data collected by the Thoraxklinik in Heidelberg, Germany.2 The dataset for this analysis included both SVS and PulmonX Zephyr valves, so this is not strictly a PulmonX-only claim. As such, it is a bit disingenuous to claim a benefit of improved mortality based on a single, retrospective study, let alone a benefit tied to a specific valve, but the data is an encouraging signal that BLVR may be associated with improved mortality, independent of valve used.
| PO2 | Treatment | Control |
|---|---|---|
| Baseline | 67.89 | 67.95 |
| Delta at 3 months | -2.34* | 0.38 |
| Delta at 6 months | -1.80* | 0.72 |
| PCO2 | Treatment | Control |
|---|---|---|
| Baseline | 40.16 | 40.94 |
| Delta at 3 months | -0.81 | -0.60 |
| Delta at 6 months | -0.59 | 0.75 |
The markedly lower pneumothorax rate in our study may be attributable to a very conservative post-operative care regimen, with patients staying in-hospital for a median of 6 days post-intervention and exposed to limited activities of daily living, which has been previously shown to reduce pneumothorax incidence.
In this study, the average emphysema severity in the ipsilateral lobe was 34.6%, with an emphysema heterogeneity between lobes of 28.4% (see Table 2). In contrast, the ipsilateral lobe emphysema severity in the TRANSFORM and LIBERATE studies was 45.4% and 47.5%, respectively, and this may account for the comparatively lower pneumothorax rate seen in the REACH study. Gompelmann, et al2., have also noted that with greater emphysematous destruction of the untreated ipsilateral lobe there is an increased incidence of pneumothorax.
Dr. Criner at Temple recently presented his order of importance when selecting a target lobe. That list, in order of priority is:
- Fissure integrity (Collateral ventilation)
- Emphysema destruction
- Perfusion
- Volume
- Degree of heterogeneity
- Anatomy
- REACTIVE ONLY: During the EMPROVE study an overnight stay was required. If the investigator had a high suspicion of potential pneumothorax, they were allowed to keep the patient longer, per their medical discretion. The mean hospital stay in EMPROVE was 3.81 days, with a median of one day.
Olympus has designated services and programs available to assist you with all of your reimbursement questions and needs related to the Spiration Valve System.
Contact Information:
Hours: 8:30 am – 5:00 pm Eastern Standard Time
Phone: 877-205-1532
Email: olympusunite@priahealthcare.com
Need Basic Reimbursement Information?
Feel free to call or email us and our trained staff can assist you with questions on billing, coding, and reimbursement for the Spiration Valve System.
Experienced coding experts are available to help providers navigate your case-specific denials and prior authorizations. Our experts can assist with communication and paperwork between your payers, and then update you on case-specific decisions through a secured provider portal.
If you are interested, please contact the Spiration Reimbursement Helpline to learn more about this service.
NOTE: A signed Business Associates Agreement is required for this level of service.
2. Gompelmann D, et al. Respiration. 2019;97(2):145-152. doi: 10.1159/000492274.
3. Li S, et al. Respiration. 2018:1-12. doi:10.1159/000494327
Download Resources
Instructional Videos
Prescriptive Information
Management of Prolonged Airleaks
Referring
Physicians
Patient
Information
- Criner GJ et al., 2019 Improving Lung Function in Severe Heterogenous Emphysema with the Spiration® Valve System (EMPROVE): A mliticenter, Open-Label, Randomized, Controlled Trial. Am J Respir Crit Care Med, 2019. https://doi.org/10.1164/rccm.201902-0383OC
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD, 2019. http://golcopd.org
- https://www.nice.org.uk/guidance/ipg600/resources/endobronchial-valve-insertion-to-reduce-lung-volume-inemphysema-pdf-1899873854992069. Accessed 2018.
- Slebos DJ, Shah PL, Herth FJ, et al. Endobronchial Valves for Endoscopic Lung Volume Reduction: Best Practice Recommendations from Expert Panel on Endoscopic Lung Volume Reduction. Respiration. 2017;93(2):138-150.
- Schuhmann M, Raffy P, Yi Y, et al. CT Predictors of Response to Endobronchial Valve Lung Reduction Treatment: Comparison with Chartis. Am J Respir Crit Care Med 2015; 191(7):767-774; doi:10.1164/rccm.201407-1205OC.
- Hogarth DK, Delage A, Zgoda MA, Reed MF. American Thoracic Society International Conference Abstracts. American Thoracic Society; 2018:A7754-A7754. doi:10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A7754.
- Wood DE, Nader DA, Springmeyer SC, et al. The IBV Valve trial: a multicenter, randomized, double-blind trial of endobronchial therapy for severe emphysema. J Bronchology Interv Pulmonol. 2014;21(4):288-297. doi:10.1097/LBR.0000000000000110.
- Criteria to determine eligible patients for the treatment are based upon findings established by the EMPROVE clinical trial. These recommendations are not meant to replace patient-specific clinical judgement. SeleCT report should not be considered a complete radiological analysis.
- Gerard Criner, MD, FACP, FACCP, the authoring physician of this video, is a paid consultant to Olympus Corporation of the Americas.
- Kirk G. Voelker, MD, the authoring physician of this video, is a paid consultant to Olympus Corporation of the Americas. Spiration is a registered trademark of Olympus Respiratory America.
- Jason Steinecker, DO, the authoring physician of this presentation, is a paid consultant to Olympus Corporation of the Americas.
- Michael Reed, MD and Jennifer Toth, MD, the authoring physicians of this presentation, are paid consultants to Olympus Corporation of the Americas.